Following your recent MRI scan and consultation with your spinal surgeon, the possibility of undergoing lumbar spinal interbody fusion has been discussed with you. This is an operation where the intervertebral disc, the structure between the bones of the spine (vertebrae), is removed and the space fused with a bone graft.
The healthy intervertebral disc acts as both a spacer and a shock absorber and is composed of two parts: a soft gel-like middle (nucleus pulposus) surrounded by a tougher fibrous wall (annulus fibrosus).
Overhead view of an intervertebral disc (simplified)
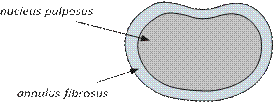
Sometimes the intervertebral discs can lose their flexibility, elasticity and shock absorbing characteristics and the tough layer of ligaments that surrounds the disc may weaken and no longer be able to contain the gel-like substance in the centre. This disc degeneration can cause inflammation in the surrounding area and some of these discs can be a source of continuing back pain and pain in the thighs and buttocks, stiffness, muscle tightness and tenderness. This is known as discogenic pain (pain arising from the disc) and is diagnosed following investigations such as an MRI, CT SPECT or a discography.
Occasionally, as a result of the continued disc degeneration, the intervertebral disc can protrude because the tough fibrous wall weakens and is therefore no longer able to contain the gel-like substance in the centre. This material may bulge or push out through a tear in the disc wall (herniation) causing pain when it touches a nerve. Lumbar nerve root pain (often called sciatica) generally goes below the knee and is felt in the area of the leg that the particular spinal nerve supplies. Symptoms also associated with sciatica include altered sensation, pins and needles, burning, numbness or even weakness of the muscles in the leg that the nerve supplies.
Treatment varies depending on the severity of the condition. Most patients only require treatment such as physiotherapy and medication, combined with some lifestyle changes or less extensive surgery, such as disc decompression. For patients whose pain does not settle with treatment or surgery, lumbar fusion surgery may be necessary. Surgery for lower back pain caused by degenerative disc disease is only considered an option for patients who:
- Have not had sufficient pain relief from extensive non-surgical treatment (such as physiotherapy, medications and pain management programmes) for at least a year;
- Have recurrent disc protrusions;
- Have ongoing lower back pain that limits their ability to perform everyday activities at work or at home; or
- Have received a diagnosis that a specific disc is the pain generator and other possible causes of the lower back pain have been considered and ruled out.
- Have a diagnosis that the leg or back pain is related to a forward slip (Listhesis) of vertebra (one of the bones of the spine). This is usually due to wear and tear or a bony defect in the bridge of bone (pars) between the joints at the back of the spine when a vertebra slips forward (spondylolisthesis), it may put pressure on a nerve which could cause leg pain or back pain.
The decision to have a lumbar interbody spinal fusion operation to treat lower back pain alone (without leg pain or nerve compression) caused by degenerative disc disease is a very personal one. For the most part, degenerative disc disease is a non-progressive type of back condition and for the majority of people their symptoms will improve over time (up to 10 years). Patients need to carefully consider the risks and possible complications along with the potential benefits of surgery, as well as consider the full range of alternatives to interbody fusion surgery.
Technically, there is a wide variety of surgical procedures that can be performed to fuse the spine, including different cages or spacers to insert into the disc space with a bone graft. The approach to the spine can also vary but in this case will be from the back, either by posterior lumbar interbody fusion (PLIF) or transforaminal lumbar interbody fusion (TLIF).
Another option which your surgeon may consider is an anterior lumbar interbody fusion (ALIF).
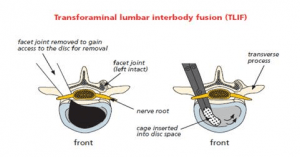
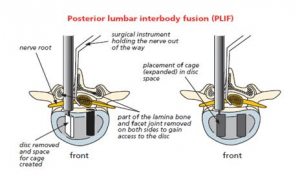
Once the diagnosis and the decision to undergo spinal interbody fusion have been made, the goal is then to obtain a solid fusion and stop the movement at that level.
The Operation
The operation is performed under general anaesthetic so you are fully asleep. First, an incision is made in the midline of your back and the muscles are lifted off the bony arch (lamina). Sometimes there may be incisions either side of the midline instead.
An internal system of screws and rods will be necessary to aid stability while the fusion takes place. These are called pedicle screws because they are placed into the part of the vertebral body of the same name, which goes directly from the back of the spine to the front. They are placed on both sides of the vertebra, above and below the disc space. These screws then act as firm anchor points to which rods can be connected. This construction then prevents movement at the part of the spine being fused.
A high-speed burr (like a dentist’s drill) is used to go through the bone to gain entry into the spinal canal. The bone is clipped away as required and the disc is then removed, right back to the bone edge of the vertebral body (end plates). Bone graft is then placed in the space created; either contained in a small metal cage or in between a metal spacer and also laid between the outer segments of the spine in between the transverse process (inter-transverse region). Your own bone will, over time, grow into the bone graft. There are several techniques to get the bone graft needed for spinal fusion:
- donor bone (allograft bone). This eliminates the need to use your own bone. The donor bone graft acts as a calcium scaffolding which your own bone grows into and eventually replaces; or
- patient’s own bone (autograft bone). The bone that is removed during surgery can be used as a bone graft. If more is needed, then it is usually taken from the pelvis (iliac crest);
- it is also possible to use artificial bone (bone substitutes).
Intervertebral fusion cage: This is like a hollow Lego brick which props up the space created between the two bones (vertebra). It is a tight fit and gives immediate stability. The cage is available in different width, height and depths to fit your spine. It is made from carbon fibre, PEEK (reinforced plastic) or titanium metal. The cage can be filled with bone graft or artificial bone. Your surgeon may use one cage (most likely in TLIF) or 2 cages (most likely in a PLIF).
After the bone graft grows and fuses to the spine (after many months), the rods and screws are no longer needed for stability. However, most surgeons do not recommend removing them except in rare cases.
After the bone graft grows and fuses to the spine (after many months), the rods and screws are no longer needed for stability. However, most surgeons do not recommend removing them except in rare cases.
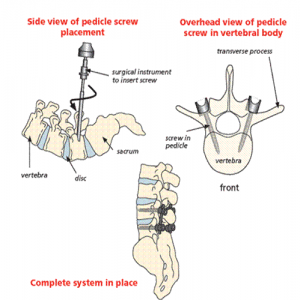
X-rays showing PLIF cages in place, side and front views
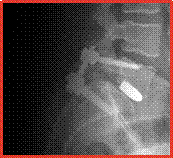
X-ray showing TLIF cage in place, side view

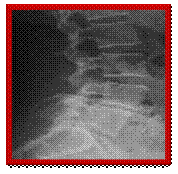
X-rays showing the before stabilisation system in place, side and front views
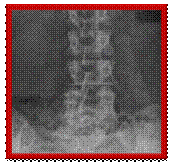
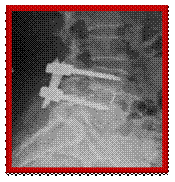
X-rays showing stabilisation system in place, side and front views Posterior lumbar interbody fusion (PLIF)

Risks and Complications
As with any form of surgery, there are risks and complications associated with this procedure.
These can include:
- damage to the nerve root and the outer lining or covering which surrounds the nerve roots (dura). This is reported in < 5% of cases (fewer than 5 out of 100 people). It may occur as a result of the bone being very stuck to the lining and tearing it as the bone is lifted off. Often the hole or tear in the dura is repaired with stitches or a patch. This could result in back or leg pain, weakness or numbness, leaking from the wound, headaches or, very rarely, meningitis;
- leg pain (sciatica) as a result of scarring around the nerve root;
- problems with positioning during the operation which might include pressure problems, skin and nerve injuries and eye complications including, very rarely, blindness. A special gel mattress and protection is used to minimise this;
- Superficial wound infections may occur in 2 – 4% of cases (up to 4 out of 100 people). These are often easily treated with a course of antibiotics. Deep wound infections may occur in < 1% of cases (fewer than 1 out of 100 people). These can be more difficult to treat with antibiotics alone and sometimes patients require more surgery to clean out the infected tissue. This risk may increase for people who have diabetes, reduced immune systems or are taking steroids;
- blood clots (thromboses) in the deep veins of the legs (DVT) or lungs (PE). This occurs when the blood in the large veins of the leg forms blood clots and may cause the leg to swell and become painful and warm to the touch. Although rare, if not treated this could be a fatal condition if the blood clot travels from the leg to the lungs, cutting off the blood supply to a portion of the lung. It is reported as happening in fewer than 1 out of 700 cases. There are many ways to reduce the risk of blood clots forming. The most effective is to get moving as soon as possible after your operation. Walk regularly as soon as you are able to, both in hospital and when you return home. Perform the leg exercises illustrated in the ‘Preventing Blood Clots’ leaflet and keep well hydrated by drinking plenty of water. Ladies are also advised to stop taking any contraceptive which contains the hormone oestrogen four weeks before surgery, as taking these during spinal surgery can increase the chances of developing a blood clot;
- You must inform your consultant if you are taking tablets used to thin the blood, such as warfarin, aspirin or clopidogrel. It is likely you will need to stop taking them before your operation as they increase the risk of bleeding;
- difficulty with screw placement, including injury to the nerves or screw breakage;
- bone graft non-union or lack of solid fusion (pseudoarthrosis). This can occur in up to 5% of cases (5 out of 100 people). See below for factors which can affect fusion;
- cage / implant movement can occur in up to 2 out of 100 cases, with 1 out of 100 requiring re-operation. In extremely rare cases, cage movement can cause severe damage and cauda equina syndrome (paralysis, bladder or bowel incontinence); it is vital that seek medical attention or attend the emergency department if you develop red flag symptoms which include the following;
- Numbness around the saddle area (genitals, buttocks, between the legs)
- Urinary difficulties such as poor flow, urgency, retention of urine, loss of sensation when urinating
- Sciatica-like pain in both legs, or weakness in the legs
- Sexual dysfunction or loss of sensation
- although rare, the surgery may make your symptoms worse than before;
- ongoing pain. Fusion surgery is a complex procedure and not all patients get complete pain relief; and
- there are also very rare but serious complications that in extreme circumstances might include damage to the cauda equina and paralysis (the loss of use of the legs, loss of sensation and loss of control of the bladder and bowel) See red flag symptoms above. This can occur through bleeding into the spinal canal after surgery (a haematoma). If an event of this nature was to occur, every effort would be made to reverse the situation by returning to theatre to wash out the haematoma. Sometimes, however, paralysis can occur as a result of damage or reduction of the blood supply of the nerves or spinal cord and this is unfortunately not reversible; and a stroke, heart attack or other medical or anaesthetic problems, including death, which is reported as happening in 1 out of 250,000 cases under general anaesthetic.
Factors which may affect spinal fusion and your recovery
There are a number of factors that can negatively impact on a solid fusion following surgery, including:
- smoking;
- diabetes or chronic illnesses;
- obesity;
- malnutrition;
- osteoporosis;
- post-surgery activities (see note on recreational activities); and
- long-term (chronic) steroid use.
Of all these factors, the one that can compromise fusion rate the most is smoking. Nicotine has been shown to be a bone toxin and inhibit the ability of the bone-growing cells in the body (osteoblasts) to grow bone. Patients should make a concerted effort to allow their body the best chance for their bone to heal by not smoking.
What to expect after surgery and going home?
Immediately after the operation you will be taken on your bed to the recovery ward where nurses will regularly monitor your blood pressure and pulse. Oxygen will be given to you through a facemask for a period of time to help you to recover from the anaesthetic. You will have an intravenous drip until you are able to drink adequately.
A drain (tube) may be placed near the surgical incision if there has been significant bleeding during the operation. This removes any excess blood or fluid collecting under the wound. The drain will be removed when the drainage has stopped, usually the next day, after surgery.
It is very normal to experience some level of discomfort or back and leg pain after the surgery. The nursing and medical staff will help you to control this with appropriate medication. The symptoms in your legs may fluctuate due to increased swelling around the nerves. As the nerves become less irritated and swollen, your leg pain should settle. This can take eight weeks, or longer. Normal feeling and strength in your legs is likely to take a lot longer and is likely to improve slowly over the next year or so. It is important not to suddenly stop taking certain pain relief medication. It may be necessary to gradually ‘wean’ yourself off them – your GP or consultant can advise you if necessary.
The physiotherapist will visit you after the operation to teach you exercises and help you out of bed. They will show you the correct way to move safely. Once you are confident and independently mobile, you will be encouraged to practise climbing stairs with the physiotherapist. Once stable, you will be allowed home, usually 1 – 2 days after surgery.
You will be giving anti-embolism compression stockings (TED) which you would have worn for your surgery, we advise to wear these for 7 days postoperatively whilst you are likely to be least mobile. This may be adjusted for a longer/shorter duration if you are not fully mobile or mobilising fully sooner.
Please arrange for a friend or relative to collect you, as driving yourself or taking public transport is not advised in the initial stages of recovery.
Wound care
Options for skin wound closure depends on your surgeon’s preference, and include absorbable sutures (stitches), removable sutures or clips (surgical staples).
If you have removable sutures or clips, you will be seen by a nurse to remove them usually 10–14 days after the operation in the outpatient department.
If you have absorbable sutures, these may need to be trimmed and a will still require a wound check to take place. The ward staff or consultant secretary will inform you of the date for your appointment or if you are required to make an appointment with your GP practice nurse.
You may shower 48 hours after surgery if you are careful, but you must avoid getting the wound wet. Most dressings used are ‘splash-proof’, but if water gets underneath, it will need to be changed. A simple, dry dressing from a pharmacy is sufficient to use if a new dressing wasn’t provided on discharge, please make an appointment in this instance for a wound check and re-dressing with outpatients’/specialist nurse. Bathing should be avoided for a minimum of two weeks.
Please contact Nuffield hospital if you think your wound might be infected. Symptoms could include:
- redness around the wound;
- wound leakage;
- a high temperature or
- Increased pain at wound site
At a later stage once the wound has healed and been checked, if the scar is sensitive to touch, you can start to massage around the scar using an unperfumed cream or oil to encourage normal sensation and healing.
Driving
Normally you will be advised to avoid driving for around four weeks (or when you no longer require the brace if you have been provided with one), but this can be adjusted depending on your recovery.
If you have no altered sensation or weakness in your legs, then you may resume driving if you feel safe to do so but you must be confident to do an emergency stop.
It is advisable not to travel for long distances initially (no longer than 20 minutes), without taking a break to ‘stretch your legs’. Gradually increase your sitting tolerance over 4 – 8 weeks.
Recreational activities
It is important to keep mobile after surgery. You will find you get stiff if sitting for longer than about 20 minutes, so get up and walk about regularly. Walking outside is fine but again, increase your walking distances gradually.
You will be advised to avoid lifting anything heavy, certainly for the first few weeks, but maybe for as long as three months, after your operation.
Please check with your consultant and physiotherapist when you are able to resume specific activities, such as swimming or golf, as the advice could range from between six weeks to three months. A gradual return to sport is then advisable. You should avoid flying for six weeks (and long-haul flights for up to three months).
Work
Returning to work is dependent on both your recovery and your job. Most people are off work for an initial four weeks, but if you are in a strenuous job you may need up to eight weeks, again this may be adjusted earlier or later and according to your recovery or type of work. It is always sensible to discuss with your employer if you can return on ‘light duties’ or reduced hours at first. There is usually nothing to stop you doing computer / office work at an earlier date, as long as you can keep moving about. The hospital will issue you with a fitness to work (sick) certificate, you can request this whilst you are in hospital or can call the ward if you have been discharged, furthermore you can request from your GP.
Follow-up
Your surgeon will advise you when you should attend clinic after your operation. If you have any queries before your follow-up date, please do contact the nurse specialist or other member of your consultant’s team.
If you have any questions regarding the information in this booklet, please discuss them with either the ward nurses or a member of your consultant’s team.

What is the British Spine Registry (BSR)?
The British Spine Registry aims to collect information about spinal surgery across the UK. This will help us to find out which spinal operations are the most effective and in which patients they work best. This should improve patient care in the future.
The Registry will enable patient outcomes to be assessed using questionnaires. These will allow surgeons to see how much improvement there has been from treatment.
This has worked for hip and knee joint replacements through the National Joint Registry. We need your help to improve spinal surgery in the UK.
What data is collected?
Your personal details allow the BSR to link you to the surgery you have had. They also allow us to link together all the questionnaires you complete. If you need any further spinal surgery in the future, details of previous operations will be available to your surgeon.
Personal details needed by the BSR are your name, gender, date of birth, address, email address and NHS number.
Your personal details are treated as confidential at all times and will be kept secure. This data is controlled by the British Association of Spine Surgeons (BASS) and held outside the NHS. Personal details will be removed before any data analysis is performed, retaining only age and gender. Your personal data and email address will not be available to anyone outside BASS and its secure IT provider. Anonymised data may be released to approved organisations for approved purposes, but a signed agreement will restrict what they can do with the data so patient confidentiality is protected.
Your personal data is very important, as this will allow us to link details of your diagnosis and surgery with any problems or complications after surgery. You may also be asked to complete questionnaires before and after surgery to work out how successful the surgery has been. These will only be possible if we can connect you to the questionnaires through your personal details.
Do I have to give consent?
No, your participation in the BSR is voluntary and whether you consent or not, your medical care will be the same. Your personal details cannot be kept without your consent. This will be obtained either by asking you to physically sign a consent form or electronically sign one through an email link to a questionnaire or at a questionnaire kiosk in the outpatient clinic.
You can withdraw your consent at any time or request access to your data by:
- Going to the patient section of the BSR website at www.britishspineregistry.com; or
- Writing to us at the BSR centre. Please state if you are happy for us to keep existing data, but do not want to be contacted, or whether you want your data to be anonymised (so it cannot be identified).
Research
Your consent will allow the BSR to examine details of your diagnosis, surgical procedure, any complications, your outcome after surgery and your questionnaires. These are known as ‘service evaluations’ or ‘audits’.
Operation and patient information, including questionnaires in the BSR, may be used for medical research. The purpose of this research is to improve our understanding and treatment of spinal problems. The majority of our research uses only anonymised information which means it is impossible to identify individuals. From time to time, researchers may wish to gather additional information. In these cases, we would seek your approval before disclosing your contact details. You do not have to take part in any research study you are invited to take part in and saying no does not affect the care you receive.
All studies using data from the Registry will be recorded on the
BSR website www.britishspineregistry.com
Further information
The BSR website at www.britishspineregistry.com contains more information, including details of any studies and any information obtained through the Registry data.
To contact the BSR, write to: The British Spine Registry
Amplitude Clinical Services
2nd Floor Orchard House
Victoria Square
Droitwich
Worcestershire WR9 8QT
